Releasing the brakes on the immune system to enhance personalized treatment of pancreatic cancer
Jacob L. Houghton, Ph.D., Discovery Grant 2017
Background and rationale:
Metastatic pancreatic cancer is the fourth leading cause of cancer death in the USA with more than 43,000 deaths per year. The current standard treatment is chemotherapy, which is very harsh and ultimately fails to control the disease, which is universally fatal. Immunotherapy (a.k.a. immune checkpoint inhibition) is a revolutionary new approach to cancer treatment that releases the brakes on the immune system, unleashing the body’s natural immunity against cancer cells. However, using an immunotherapy drug does not work well as a standalone therapy for pancreatic cancer. In the same vein, external beam radiation therapy, in which the tumor is directly targeted with radiation, has proven largely successful in many cancer types but not pancreatic cancer. Independently, these two therapies are not successful for metastatic pancreatic cancer, so it may seem surprising, then, that the goal of this project is to develop a treatment for pancreatic cancer that combines radiation and immunotherapy. Rest assured; there is a strong rationale for this approach.
It has been shown in many cancer types that radiation therapy not only kills tumor cells in the vicinity of the radiation, but it also triggers a robust immune response. In some cases, this immune response even leads to a therapeutic effect in metastases that were not directly exposed to radiation, which is known as an “abscopal effect.” There is strong evidence that unshackling the immune system via immunotherapy could synergistically enhance this so-called abscopal effect in many cancer types, including pancreatic cancer. In fact, one of my collaborators at Vanderbilt University Medical Center was the first to demonstrate this synergism in metastatic, castrate-resistant prostate cancer. However, nobody has investigated this approach for pancreatic cancer. Based on precedence and a large body of scientific evidence, I hypothesize that combining immunotherapy and radiation therapy will be highly effective in pancreatic cancer. To methodically test this hypothesis, there is one major hurdle that will need to be overcome. That is, not everyone’s cancer cells can be targeted by our specific immunotherapy, and those who do not are unlikely to respond. As such, we need a “personalized medicine” approach to select our patients.
Approach:
To identify patients who might benefit from our approach, we will develop a medical imaging diagnostic tool (called a PET tracer) by labeling the immunotherapy drug with a radioactive isotope. After injecting this into the patient, we can use special cameras that detect radiation to visualize where our immunotherapy sticks in the patient. These images will tell us if the “biomarker” is present on the patient’s cancer cells and if they are likely to benefit from our novel treatment. This will allow us to select only those patients who are likely to respond, eliminating any false-negatives that would confound our analysis. Fortuitously, this same PET imaging procedure may be used during the course of therapy to monitor response and inform progress, as depicted in the figure below. This personalized medicine strategy would represent a huge leap forward over current practice because it typically takes six months of immunotherapy treatment before we can tell if a patient is responding. Additionally, it would prevent patients who will not respond from wasting their time on an ultimately fruitless treatment regimen.
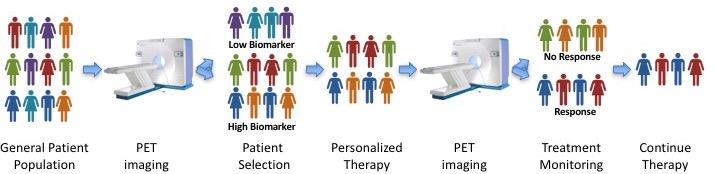
Before we can attempt this innovative strategy in patients, we must prove that this strategy works in preclinical models of pancreatic cancer. My laboratory utilizes the most advanced models of pancreatic cancer available, and we will utilize these models to test our hypothesis. We will test our combination therapy approach, comparing the response to radiation or immunotherapy alone, our primary benchmark being increased survival. Furthermore, we will test our imaging agents to ensure that we are able to select tumors with high levels of our immunotherapy target. This data will provide the rationale for proposing clinical trials in human patients as well as the preliminary data to seek funding for more in-depth studies of this phenomenon.
Impact and significance:
This approach represents a great leap forward for successfully combining immunotherapy, radiation therapy, and medical imaging for personalized cancer care. Excitingly, our groundbreaking medical imaging will be useful for the entire field of immunotherapy, not just for pancreatic cancer! In fact, my laboratory is currently working to obtain FDA approval for the immunotherapy imaging agent studies to support clinical trials in triple-negative breast cancer as well as castrate-resistant, metastatic prostate cancer. Thus, if these pilot studies are successful, we will rapidly translate our findings into the clinic, assuring that our work will provide the maximum benefit for those suffering from pancreatic cancer as soon as possible.


